Medical device manufacturing for FDA-regulated markets requires rigorous quality management systems, comprehensive documentation, and validated processes. For contract fabricators and OEMs navigating FDA compliance, understanding regulatory requirements is essential for successful market entry and sustained production.
FDA Medical Device Classification and Manufacturing Impact
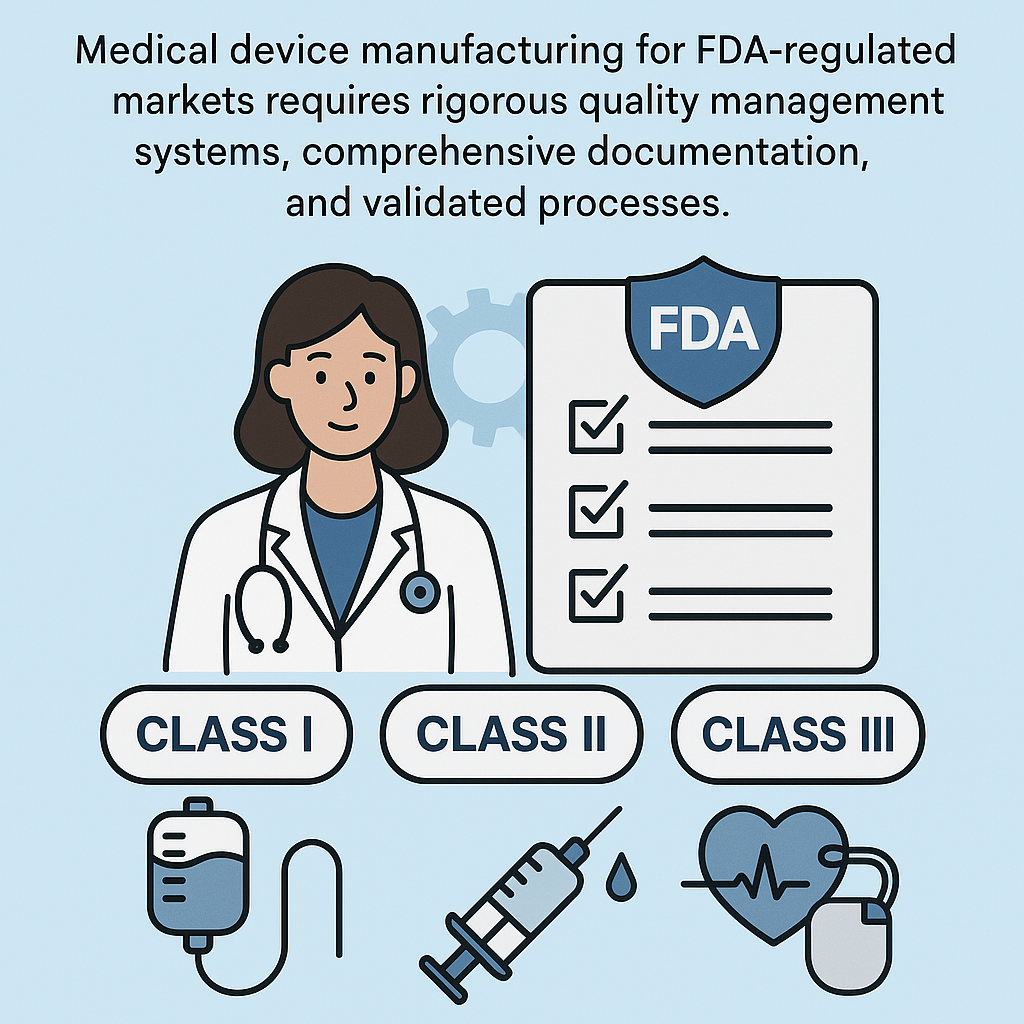 The FDA classifies medical devices into three categories based on risk level, with each classification imposing different manufacturing requirements:
The FDA classifies medical devices into three categories based on risk level, with each classification imposing different manufacturing requirements:
Class I Devices (lowest risk): Basic equipment like examination gloves and elastic bandages. Generally require FDA registration and adherence to general controls including Good Manufacturing Practices (GMP). Most are exempt from premarket notification.
Class II Devices (moderate risk): Devices like infusion pumps, surgical instruments, and patient monitoring equipment. Require FDA 510(k) premarket notification, adherence to special controls, and comprehensive quality system compliance. Manufacturers must demonstrate “substantial equivalence” to existing devices.
Class III Devices (highest risk): Life-supporting or life-sustaining devices including implantable pacemakers, coronary stents, and artificial heart valves. Require premarket approval (PMA), extensive clinical trial data, and the most stringent manufacturing controls and oversight.
Manufacturing requirements scale with device classification. Class III device fabrication demands validated processes, complete traceability, design control documentation, and extensive quality system audits.
FDA Quality System Regulation (QSR) Requirements
The FDA’s Quality System Regulation (21 CFR Part 820) establishes mandatory quality management system requirements for medical device manufacturers. Key QSR elements include:
Design Controls: Formal design and development procedures with documented planning, input requirements, output specifications, design review, verification, validation, and design transfer. Essential for Class II and Class III devices.
Document Controls: Comprehensive document management systems ensuring all manufacturing procedures, work instructions, and specifications are current, approved, and accessible to relevant personnel.
Process Validation: Documented evidence that manufacturing processes consistently produce devices meeting specifications. Includes installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ) protocols.
Purchasing Controls: Evaluation and approval of suppliers, with documented evidence that purchased components meet specifications. Critical for medical device supply chain integrity.
Production and Process Controls: Written procedures for all manufacturing operations, equipment maintenance schedules, environmental controls, and personnel training requirements.
Corrective and Preventive Action (CAPA): Formal systems for identifying, investigating, and correcting quality problems. FDA audits heavily scrutinize CAPA effectiveness.
Traceability: Device history records (DHR) documenting complete manufacturing history for each production unit or batch, enabling recall capability and failure investigation.
ISO 13485 and FDA Compliance
ISO 13485 is the international standard for medical device quality management systems. While FDA QSR compliance is mandatory for U.S. market access, ISO 13485 certification demonstrates compliance with internationally recognized quality standards and facilitates global market entry.
Many medical device manufacturers pursue both FDA QSR compliance and ISO 13485 certification. ISO 13485 requirements align closely with FDA QSR but include additional risk management elements (ISO 14971) and emphasize a process approach to quality management.
Contract manufacturers serving medical device OEMs often maintain ISO 13485 certification to demonstrate quality system maturity and support customers’ regulatory compliance requirements.
Material Requirements and Biocompatibility
Medical device fabrication requires careful material selection based on intended use, patient contact, and duration of contact. FDA guidance references ISO 10993 series standards for biological evaluation of medical devices.
Material Considerations for Precision Sheet Metal Fabricators:
- Stainless steel grades: 316L most common for implantable devices and surgical instruments due to corrosion resistance and biocompatibility. 304 stainless acceptable for non-implant applications.
- Titanium alloys: Ti-6Al-4V and commercially pure titanium for implantable devices requiring strength and biocompatibility. Excellent corrosion resistance and osseointegration properties.
- Material traceability: Complete documentation of material certifications, heat lot numbers, and mechanical/chemical properties. Certificate of Conformance required for all materials.
- Surface finish requirements: Medical devices often specify precise surface finish (Ra values) to prevent bacterial adhesion, ensure cleanability, or meet functional requirements.
Cleanroom and Environmental Controls
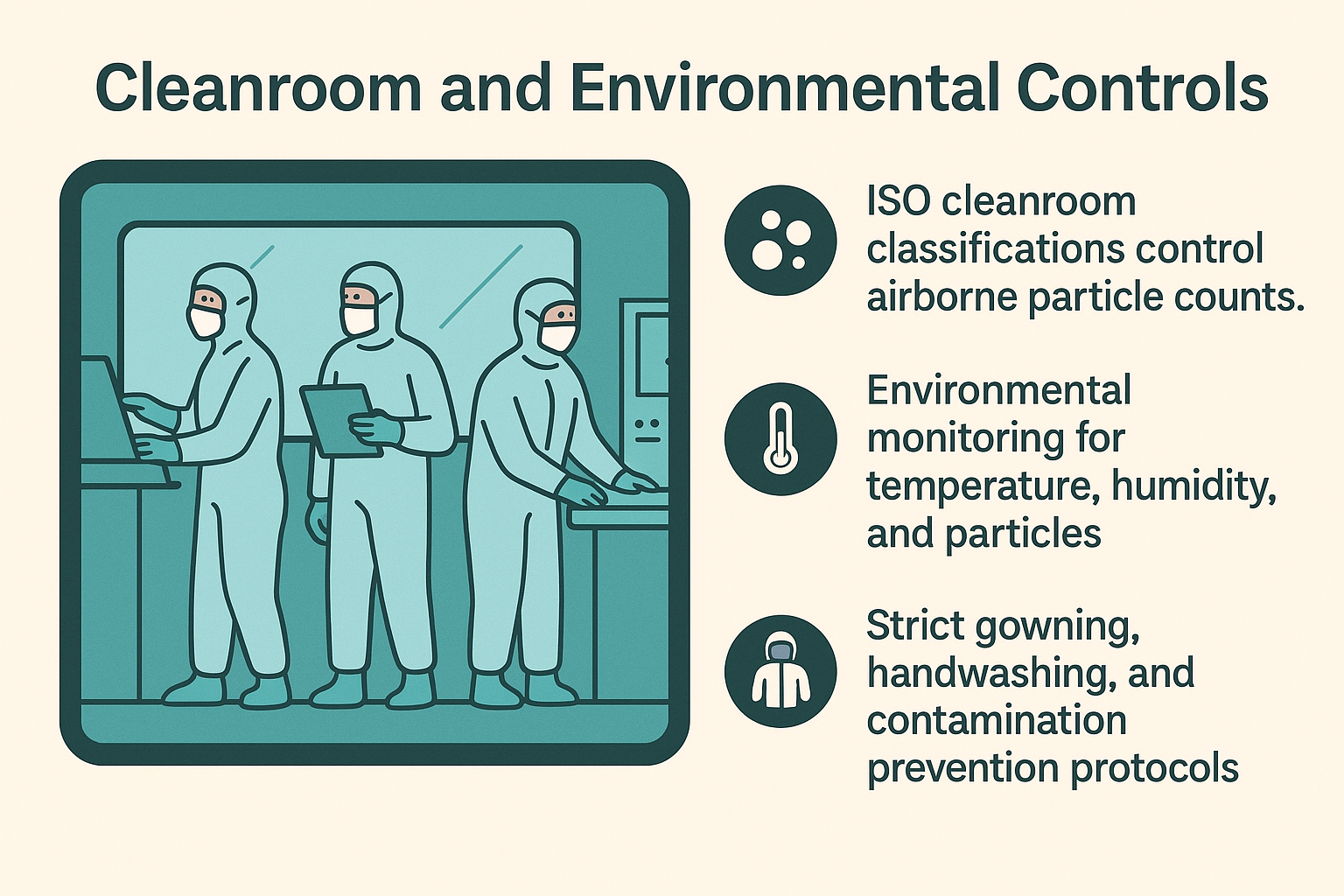
ISO Cleanroom Classifications: Ranging from ISO Class 5 (Class 100) for sterile device assembly to ISO Class 8 (Class 100,000) for non-sterile device component fabrication. Classification based on airborne particle counts.
Environmental Monitoring: Temperature, humidity, and particle count monitoring with documented acceptable ranges. Critical for maintaining validated manufacturing conditions.
Personnel Controls: Gowning requirements, hand washing protocols, training on contamination prevention, and personnel health monitoring for cleanroom operations.
Not all medical device fabrication requires cleanroom environments. Metal enclosures for diagnostic equipment or surgical instrument handles may not require controlled environments, while implantable component fabrication demands stringent controls.
Manufacturing Process Validation
FDA requires validation of processes where results cannot be fully verified by inspection and testing of the finished device. Metal fabrication processes requiring validation include:
Welding: Welding validation includes procedure qualification, welder qualification, and production weld validation. Documentation includes welding procedure specifications (WPS), procedure qualification records (PQR), and ongoing production monitoring.
Heat Treatment: Temperature uniformity surveys, load mapping, and process capability studies demonstrating consistent metallurgical properties. Critical for devices requiring specific hardness or strength characteristics.
Cleaning and Decontamination: Validated cleaning procedures with documented residue testing demonstrating removal of manufacturing contaminants (oils, cutting fluids, particles).
Sterilization (if performed): Extensive validation including bioburden testing, sterility assurance level (SAL) demonstration, and package integrity validation.
Supplier Management and Traceability
Medical device manufacturers must maintain comprehensive supplier qualification and monitoring programs:
Supplier Qualification: Assessment of supplier quality systems, capability studies, and initial product qualification before approval for production use.
Incoming Inspection: Verification that purchased components meet specifications through inspection, testing, or certificate of conformance review.
Traceability: Lot number tracking for all materials and components, enabling complete genealogy documentation for each manufactured device.
Supplier Corrective Actions: Formal processes for addressing supplier nonconformances with documented corrective action plans and effectiveness verification.
FDA Facility Registration and Inspection
All medical device manufacturers must register facilities with the FDA and list marketed devices. FDA conducts periodic unannounced inspections focusing on:
- Design control effectiveness (for Class II/III devices)
- CAPA system performance
- Process validation documentation
- Supplier controls and incoming inspection
- Device history record completeness
- Management review of quality system
Inspection findings are documented on FDA Form 483. Manufacturers must respond with corrective action plans addressing each observation. Warning letters may result from serious or repeated violations.
 Contract Manufacturing Considerations
Contract Manufacturing Considerations
Medical device OEMs partnering with contract manufacturers should verify:
Quality System Maturity: ISO 13485 certification, FDA establishment registration, history of successful FDA inspections without warning letters.
Technical Capabilities: Equipment qualifications, validated processes, material handling procedures, and cleanroom facilities (if required).
Documentation Systems: Capability to maintain device master records (DMR), device history records (DHR), and support OEM’s regulatory submissions.
Change Control: Formal processes ensuring manufacturing changes are evaluated for impact on device safety/performance with OEM notification and approval.
Practical Implications for Your Medical Device Projects
When evaluating metal fabrication partners for FDA-regulated device manufacturing:
Expect comprehensive documentation: Every process parameter, material certification, and inspection result will be documented. This creates traceability but increases administrative burden.
Plan for validation activities: Initial process validation requires significant time and investment. Factor 6–12 weeks for comprehensive validation protocols.
Understand material premiums: Medical-grade materials with full traceability cost 20–40% more than commercial grades. Certificate of Conformance is mandatory, not optional.
Budget for quality system overhead: FDA-compliant manufacturing includes quality system costs (document control, CAPA, training, audits) that increase unit costs 15–25% versus commercial production.
Allow for longer lead times: Design review, process validation, and documentation requirements extend initial production timelines. Plan 8–16 weeks for first article production versus 4–6 weeks for commercial components.
EVS Metal’s Medical Manufacturing Capabilities

- ISO 9001:2015 certified quality management system with document control, CAPA, and supplier management infrastructure
- Advanced manufacturing equipment with documented maintenance schedules and calibration programs
- Material traceability systems tracking certifications and lot numbers through production
- Welding procedure qualification and certified welders for critical applications
- First article inspection and dimensional verification capabilities supporting tight tolerance requirements
While EVS does not currently maintain ISO 13485 certification, our quality systems infrastructure and manufacturing capabilities support medical device OEMs requiring metal fabrication components with comprehensive documentation and traceability.
Considering medical device component manufacturing? Request a quote to discuss your FDA compliance requirements and manufacturing specifications, or call EVS Metal at (973) 839-4432.
Frequently Asked Questions
How does the FDA classify medical devices?
The FDA classifies medical devices into three categories based on risk. Class I devices are low risk and generally require registration and adherence to general controls. Class II devices are moderate risk and typically require 510(k) premarket notification, special controls, and a compliant quality system. Class III devices are high risk, life-supporting or life-sustaining, and require premarket approval (PMA), clinical data, and the most stringent manufacturing controls.
What is the FDA Quality System Regulation (QSR)?
The FDA Quality System Regulation, 21 CFR Part 820, defines mandatory quality management system requirements for medical device manufacturers. It covers design controls, document control, process validation, purchasing controls, production and process controls, CAPA, and traceability. Compliance with QSR is required for FDA-regulated medical devices marketed in the United States.
Does my contract manufacturer need ISO 13485 certification to fabricate medical device components?
Not necessarily. ISO 13485 certification demonstrates quality system maturity but isn’t mandatory. The medical device OEM (you) maintains regulatory responsibility. Your contract manufacturer must meet your specified quality requirements and provide documentation supporting your regulatory submissions. Many OEMs successfully work with ISO 9001-certified fabricators for lower-risk components.
What’s the difference between commercial-grade and medical-grade stainless steel?
Chemically they’re identical—316L is 316L. The difference is documentation and traceability. Medical-grade material includes full mill test reports, heat lot numbers, and complete chemical/mechanical property certification. This traceability enables lot-specific recalls and failure investigation if needed.
How much does FDA compliance add to manufacturing costs?
FDA-compliant manufacturing typically adds 15–30% to unit cost compared to commercial production. Cost drivers include material traceability and documentation, process validation activities, enhanced quality system controls, and additional inspection and record-keeping requirements. Higher-risk Class III device components may carry even greater premiums due to more stringent controls.
Can the same components be used for commercial and medical versions of a product?
Physically, the same design can often be used for both commercial and medical versions of a product, but the documentation requirements differ. Medical device components must have full traceability, device history records, and validated processes. Most manufacturers assign separate part numbers for medical versus commercial versions to keep documentation, control plans, and traceability clearly separated.
What happens if there is a manufacturing defect in a medical device component?
If a manufacturing defect is discovered, the medical device OEM must use its CAPA system to investigate root cause, assess risk to patients, and determine whether field action is required. Possible actions include corrections, recalls, or notifications to regulators. Complete traceability through device history records and lot tracking is essential to identify affected devices and respond effectively.
How long does initial process validation take for medical device manufacturing?
Initial process validation typically takes 6–12 weeks and includes protocol development, equipment qualification, process capability studies, and final validation reporting. Compressed timelines of 4–6 weeks are sometimes possible but can increase risk if validation is incomplete or insufficient to withstand regulatory scrutiny.
Why does medical-grade material and documentation increase lead time?
Medical-grade material and documentation requirements extend lead time because they require controlled sourcing, full certification packages, and additional review. Combined with design review, process validation, and first article inspection, initial medical device builds often require 8–16 weeks, compared to 4–6 weeks for comparable commercial components.
What capabilities should a metal fabrication partner have for FDA-regulated devices?
A suitable metal fabrication partner for FDA-regulated devices should have a robust quality management system, document control, CAPA processes, material traceability, validated special processes such as welding or cleaning, and comprehensive inspection capabilities. Experience with medical device documentation, device history records, and support for OEM regulatory submissions is also highly valuable.

 Contract Manufacturing Considerations
Contract Manufacturing Considerations